Sie befinden sich hier
Inhalt
About Our Research Focus
The Stellos Lab is located at the European Center for Angioscience of the Heidelberg University in Mannheim, Germany. Our research focuses on the regulatory role of RNA in vascular endothelial control of age- and metabolic dysfunction-related diseases. Specifically, we investigate how inflammatory, metabolic, and RNA-biological mechanisms drive vascular and multi-organ disease.
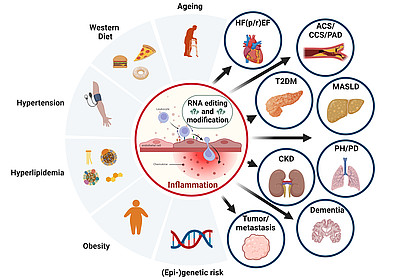
The accompanying figure illustrates how ageing, hypertension, dyslipidemia, obesity, Western diet, and (epi)genetic risk converge on endothelial inflammation and dysregulation of RNA editing and modification. These processes promote cardiometabolic, renal, pulmonary, oncologic, and neurodegenerative diseases. Our mission is to translate these mechanisms into improved diagnostics and therapeutic strategies for patients.
1. RNA in Systems Biology and Medicine
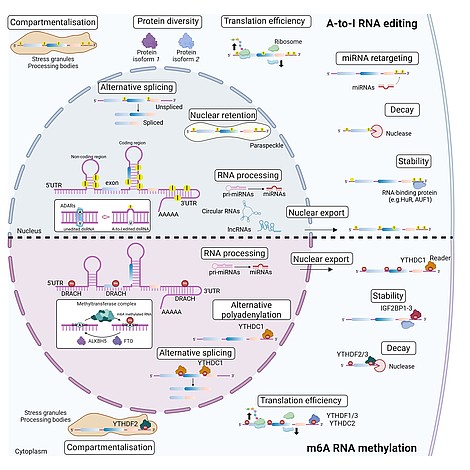
Our research investigates how RNA editing and epitranscriptomic modifications function as dynamic regulatory layers that shape endothelial behavior, modulate immune cell activation, and drive vascular remodeling across cardiometabolic, inflammatory, and age-associated diseases. Building on the lab’s strong foundation in cardiovascular RNA biology, we combine multi-omics profiling of deeply phenotyped patient cohorts with mechanistic studies in human endothelial, hematopoietic, and immune cell systems. This integrated systems-level strategy allows us to map disease-associated RNA editing signatures, RNA modification patterns, and non-coding RNA networks that govern transcriptional plasticity, intercellular communication, and vascular homeostasis.
Using state-of-the-art technologies—including long-read RNA sequencing, direct RNA modification mapping, high-resolution imaging, and perturbation-based functional genomics—we identify RNA-driven signatures linked to disease progression, inflammatory activation, and therapeutic response. A particular focus is placed on uncovering causal RNA regulators, such as ADAR-mediated editing events, m⁶A-dependent control of transcript fate, and RNA–protein interactions that orchestrate cellular adaptation to metabolic and inflammatory stress.
By bridging fundamental mechanistic insights with clinically grounded research questions, our lab aims to transform RNA biology into actionable biomarkers, predictive tools, and innovative RNA-targeted therapeutic strategies. Ultimately, our work advances a precision RNA medicine framework with the potential to reshape the prevention, diagnosis, and treatment of vascular and inflammatory disorders.
Selected publications
2. Understanding how metabolism shapes vascular health
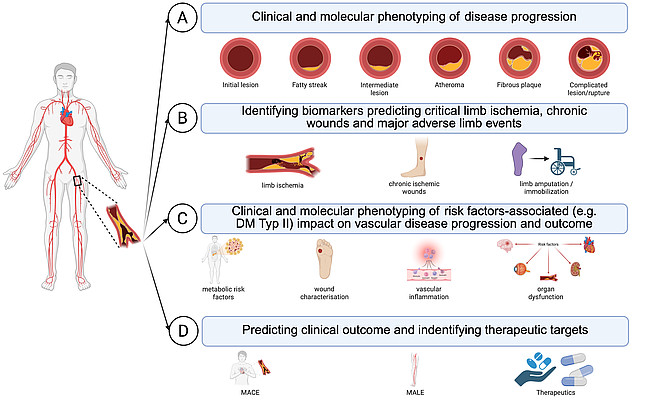
Our lab focuses on understanding how metabolic dysfunction—particularly in diabetes and obesity—drives vascular inflammation and contributes to persistent cardiovascular risk despite standard therapy. Through the Angiometabolic Study, a prospective cross-European cohort, we combine structured clinical workflows, advanced vascular imaging, and molecular profiling to generate insights that directly inform risk stratification and patient management.
Integrated Clinical Workflow
Participants undergo standardized, reproducible assessments at baseline and follow-up visits, including:
- Comprehensive clinical phenotyping and cardiometabolic risk scoring
- Biomarker-based profiling (inflammatory markers, metabolic indices)
- Longitudinal follow-up for atherosclerosis progression and major cardiovascular outcomes
- This protocol enables consistent evaluation across centers and facilitates real-world clinical translation.
Advanced Vascular Imaging
We employ a multimodal vascular imaging pipeline designed to capture disease across the arterial tree:
- High-resolution ultrasound for carotid, aorta and femoral atherosclerosis burden and plaque progression
- Pulse-wave velocity (PWV) for assessment of macrovascular stiffness
- Microvascular reactivity testing for early endothelial dysfunction
- Ambulatory and central aortic blood pressure monitoring for hemodynamic characterization
These imaging modalities provide clinicians with actionable markers of vascular health and early disease progression.
Clinical Relevance and Patient Benefit
Our integrated approach supports clinicians by:
- Identifying individuals at high residual cardiovascular risk despite guideline-directed therapy
- Detecting subclinical vascular disease before symptomatic presentation
- Improving risk prediction using combined imaging and biomarker signatures
- Enabling more personalized treatment decisions, including timing of pharmacotherapy escalation
- Informing follow-up intensity and preventive strategies
By linking mechanistic insights with real-world clinical endpoints, we aim to enhance early detection, refine patient stratification, and support precision vascular care across the cardiometabolic spectrum.
Selected publications
3. ImmunoVascular Control of Health and Disease
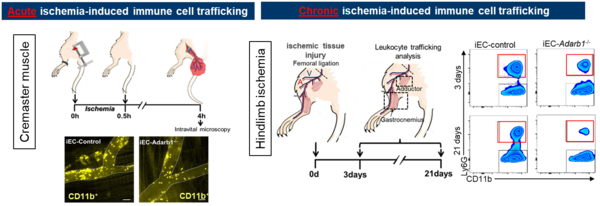
Dysregulated communication between endothelial and immune cells is one of the main drivers of vascular ageing, atherosclerosis, ischemic injury, and chronic inflammation. The Stellos Lab focuses on deciphering the mechanisms that govern immune-vascular interactions, beyond the major inflammatory pathways. Our recent work has uncovered key RNA-based regulators of endothelial activation, including the ADAR2-dependent RNA editing pathways that modulate leukocyte recruitment. Building on these findings, we now aim to integrate multi-omics profiling, computational modelling, and functional vascular biology to identify novel RNA-centered targets that can be therapeutically leveraged to restore immune-vascular homeostasis and prevent cardiovascular disease progression.
Selected publications
4. Cardiovascular Ageing & Systemic Disease
Cardiovascular ageing is a systems-level process in which vascular homeostasis progressively declines, increasing vulnerability to heart failure, kidney disease, pulmonary vascular disorders, cancer progression, and dementia. Our research programme approaches this complex biology through an integrated framework that connects vascular ageing, immune–vascular crosstalk, and protein misfolding.
A central focus of our work is amyloid-β40, traditionally viewed as a neurocentric molecule. We reposition Aβ40 as a potent vascular stressor that drives endothelial dysfunction, inflammation, atherogenesis, and arterial stiffening—reframing aspects of arterial ageing as a protein-misfolding disease.
In parallel, we investigate how ageing reshapes the metabolic and functional states of immune and stromal cells, activates transposable elements and cGAS–STING signalling, and remodels the epigenetic and epitranscriptomic landscape, pushing tissues into persistent inflammatory states.
To capture this multidimensional biology, we combine single-cell and spatial multi-omics, comparative analyses in long-lived species, engineered mouse models, and deeply phenotyped human ageing cohorts. Our goal is to translate these insights into minimally invasive biomarkers—including Aβ40—and into senotherapeutic, metabolic, anti-LINE1, and multimodal interventions that restore immune–vascular balance and slow vascular decline.
Selected publications