Sie befinden sich hier
Inhalt
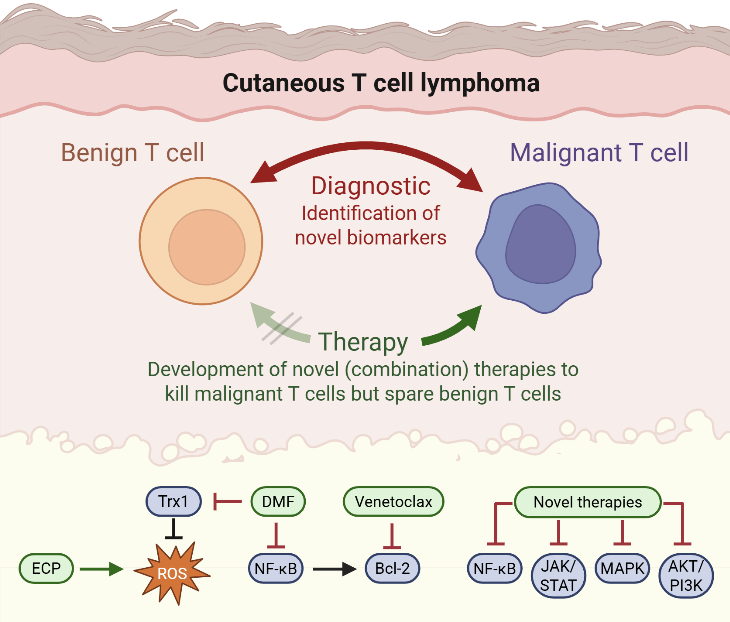
Cutaneous T-cell lymphomas (CTCL) are a group of rare diseases characterized by the presence of malignant T-helper cells in the skin, which in advanced stages can spread to the blood, lymph nodes, or internal organs. The most common CTCL subtypes are Mycosis fungoides (MF) and the more aggressive leukemic variant, Sézary Syndrome (SS).
CTCL poses significant diagnostic and therapeutic challenges:
- First, there are no unique markers that reliably distinguish malignant T cells from healthy ones, often leading to delayed or difficult diagnoses.
- Second, no curative therapies currently exist.
- Third, available treatments are frequently limited by suboptimal efficacy or poor tolerability.
These challenges underscore the urgent need for novel diagnostic and prognostic markers, which could enable earlier and more accurate detection of CTCL.
Our research addresses this need by investigating the biology of malignant T cells, focusing on identifying cellular and molecular alterations unique to these cells. These alterations are then evaluated for their potential both as diagnostic tools and as therapeutic targets based on T-cell functions.
Our work further supports the development of new therapies for T-cell–mediated diseases, including both malignant conditions like CTCL and chronic inflammatory disorders. By targeting disease-driving pathways in T cells, we aim to develop treatment strategies that are not only more effective but also better tolerated by patients.
A central component of our translational research is the initiation and conduct of investigator-initiated trials, through which we can rapidly translate laboratory findings into clinical applications. In parallel, we also participate in industry-sponsored and EORTC clinical trials, ensuring our patients have access to novel therapeutic approaches and contributing to international multicenter research efforts.
This comprehensive approach spanning basic research, biomarker discovery, therapeutic development, and clinical evaluation has already led to substantial progress. Several of our findings have been translated from bench to bedside, including the clinical implementation of targeted therapies developed from our research. These advances bring us closer to our ultimate goal: improving outcomes and quality of life for patients with CTCL and other T-cell–mediated diseases.
Research highlights
Advances in targeted therapies for CTCL
Therapy of CTCL is a great challenge due to short duration of response and limited tolerability of the available treatment options. Therefore, our research is focused on developing novel therapies that specifically target the distinct cell death resistance of CTCL cells and spare benign bystander T cells. Dysregulation of the MAPK pathway in CTCL promotes both proliferation and resistance to cell death, highlighting it as a potential therapeutic target (Kiessling*, Nicolay* et al.: Oncotarget 2017). Furthermore, we identified the NF-κB inhibitor dimethyl fumarate (DMF) as a promising candidate in preclinical studies (Nicolay JP et al.: Blood 2016). In a translational approach, we also performed a clinical phase II multicenter study to confirm the effects also in a clinical context (Nicolay et al.: Blood 2023). At the moment we develop further targeted and immunologic therapies for T cell diseases
Novel combination therapies for CTCL
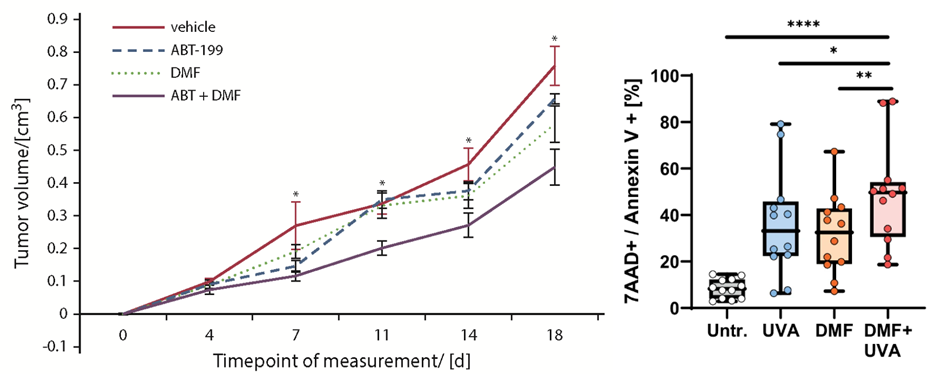
We aim to develop novel targeted combination therapies that improve both efficacy and tolerability in CTCL. In this context, we have demonstrated synergistic effects when combining DMF with Bcl-2 inhibition using venetoclax (Fröhlich et al., Blood, 2019), as well as with extracorporeal photopheresis (ECP) (Sener*, Melchers* et al., Leukemia, 2024). A clinical trial evaluating the DMF/venetoclax combination is currently in preparation. As an initial step toward clinical translation, six patients have already been treated with the DMF/ECP combination, achieving an overall response rate of 100%.
Diagnostic markers and therapeutic targets in CTCL
In order to improve and accelerate CTCL diagnosis we aim to develop novel diagnostic markers. CTCL cells differ from benign T cells by several altered signaling pathways and aberrant surface molecule expression. Here, we study malignant T cells from the skin and the blood to characterize their marker profile (Ruggiero*, Nicolay* et al.: Nature Communications 2015; Nicolay et al.: JDDG 2016, Melchers et al.: Exp Dermatol 2024). By correlation with clonality analyses and cell death resistance we can identify the markers that describe the malignant population best and thus might serve as novel therapeutic targets.
Immunologic effects of targeted therapies
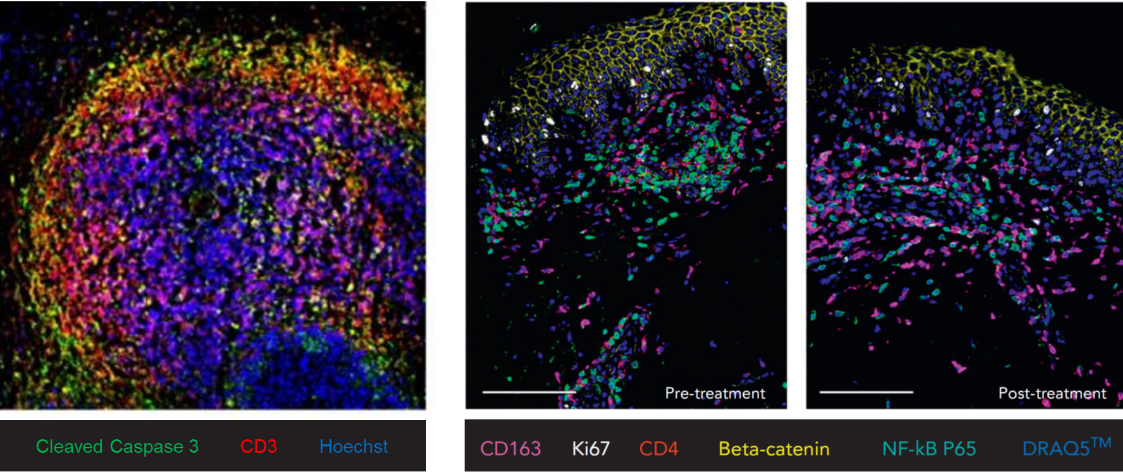
To better understand the immunologic mechanisms underlying treatment response in CTCL, we investigate changes in the tumor microenvironment following targeted therapies such as mogamulizumab. Our analyses include single-cell RNA sequencing to characterize treatment-induced shifts in immune cell composition and function. These approaches enable us to dissect therapy-related alterations at high resolution and to identify potential biomarkers of response or resistance.
Clinical studies in CTCL research
To advance new therapeutic approaches, we develop and perform clinical trials in CTCL. As one of the few centers across Europe, we participate in all ongoing multicenter CTCL trials currently investigating novel therapies for this disease group in Europe. In this context, we were involved in developing a recent multicentric EORTC trial with a financial volume of 2.5 M Euro (EORTC 1820/MOGAT). In addition, as mentioned above, we have completed our own clinical therapy study to investigate DMF, an inhibitor of the NF-κB pathway that is dysregulated in cutaneous T-cell lymphomas (Nicolay et al.: Blood 2023). Building on these findings, we are currently planning another investigator-initiated trial (IIT) exploring the combination of DMF and venetoclax.
Evaluating quality of life and response to therapy in CTCL
In order to improve the current therapeutic management of our patients, we perform several clinical investigations that accompany therapy (MINT). In these non-interventional studies, we assess the course of quality of life for our patients as well as biomarkers and response/side effects during therapy. By correlating these factors, we aim to improve patient stratification. These projects are supported by several funding organizations and industry.
